WO2018015581A1 - Integrated system for capturing co2 and producing sodium bicarbonate (nahco3) from trona (na2co3 - 2h2o - nahco3) - Google Patents

6.2g of a sample containing Na2CO3, NaHCO3 and non-volatile inert impurity on gentle heating loses 5% of its mass due to reaction 2NaHCO3 rarr Na2 CO3 + H2 O + CO2 .
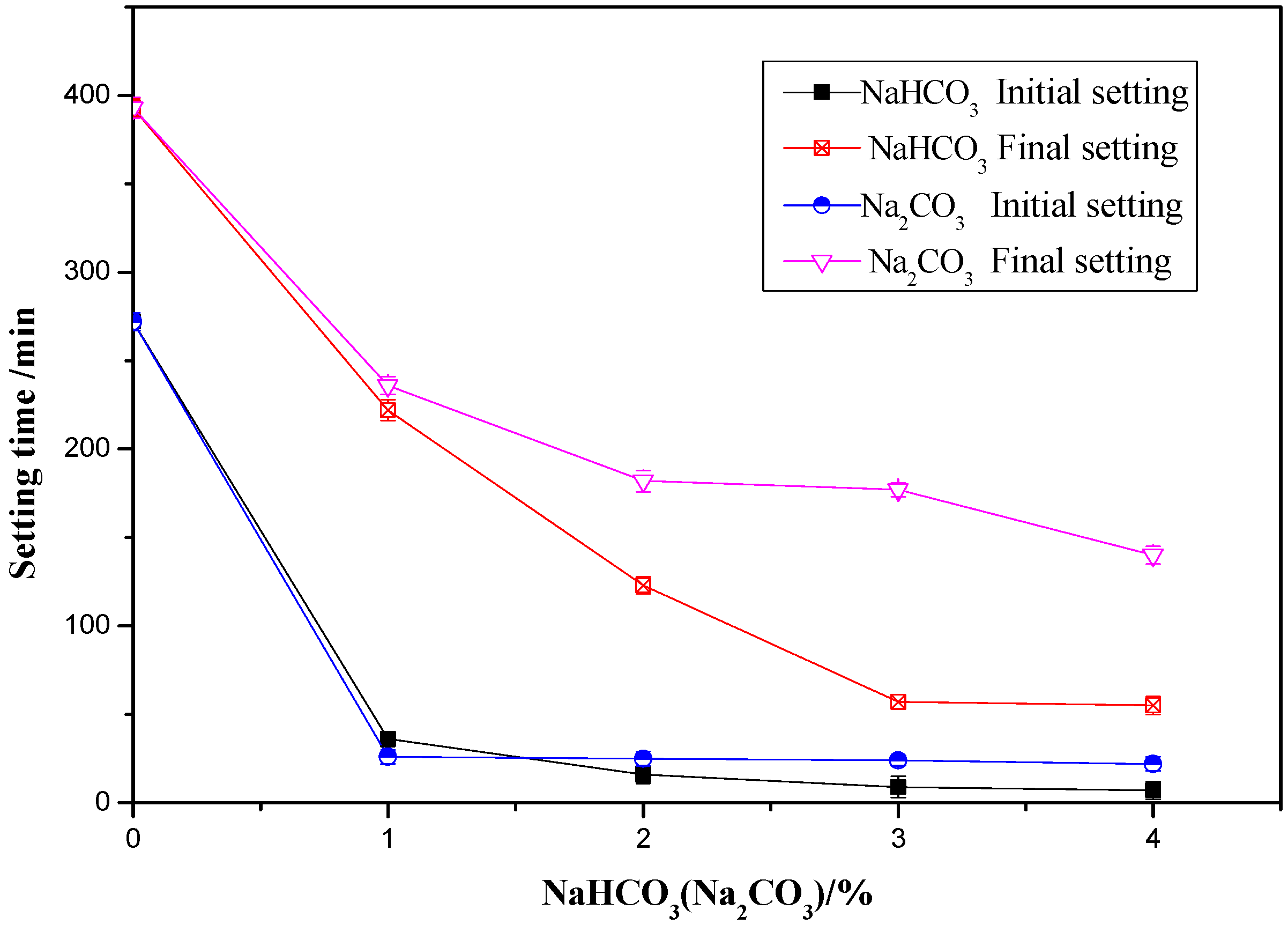
Materials | Free Full-Text | Comparison of Effects of Sodium Bicarbonate and Sodium Carbonate on the Hydration and Properties of Portland Cement Paste
31. A mixture of Na2CO3 and NaHCO3 having a total weight of 100g. On heating with O2 produces 11.2litres of CO2 under STP conditions , the percentage of Na2CO3 in that mixture

A 100ml solution contained Na2CO3 and NaHCO3 25ml of the solution required 5ml of N/10 HCl for neutalization using - Chemistry - Solutions - 13530337 | Meritnation.com

Table 2 from Solubility of ThO2·xH2O(am) and the formation of ternary Th(IV) hydroxide-carbonate complexes in NaHCO3-Na2CO3 solutions containing 0−4 M NaCl | Semantic Scholar

1 gm mixture of nahco3 and na2co3 is heated to 150 degree celcius.The volume of co2 produced is 112 ml - Brainly.in

Solid Phases and Their Solubilities in the System Na2CO3 + NaHCO3 + Ethylene Glycol + Water from (50 to 90) °C | Journal of Chemical & Engineering Data
40. A 2g sample containing Na2CO3 and NaHCO3 loses 0.248g when heated to 300^° c,the temperature at which NaHCO3 decomposes to Na2CO3,CO2 AND H2O.what is the precentage of Na2CO3 in the given



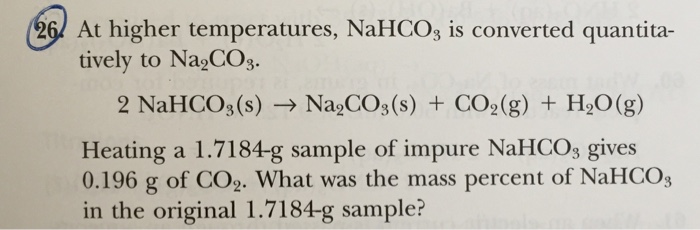






![Sodium Sesquicarbonate [Na2CO3.NaHCO3 .2H2O] [CAS_533-96-0] White Need – Wintersun Sodium Sesquicarbonate [Na2CO3.NaHCO3 .2H2O] [CAS_533-96-0] White Need – Wintersun](http://cdn.shopify.com/s/files/1/0724/7981/products/19-119-2_large.jpg?v=1655159138)