Cemiplimab-rwlc Injection, for Intravenous Use (Libtayo®) HCPCS Code J9999: Billing Guidelines | NC Medicaid

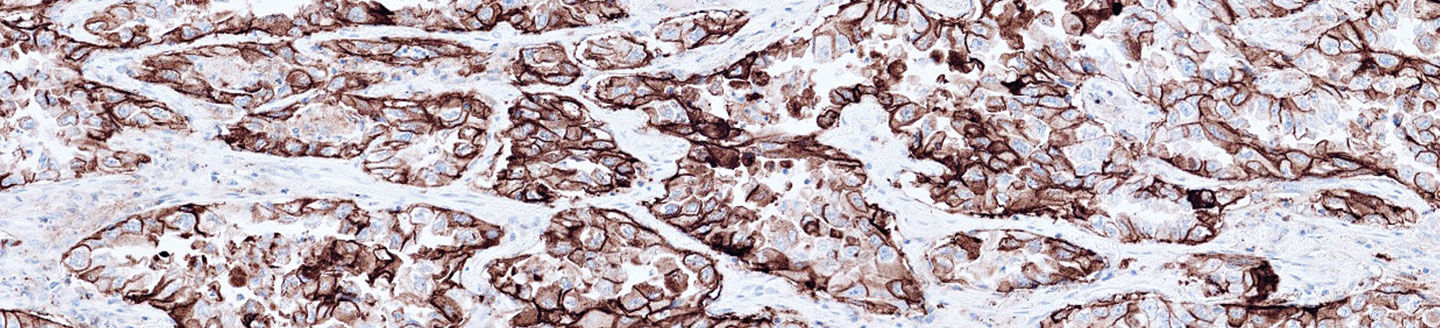
Roche's VENTANA PD-L1 (SP263) Assay receives CE IVD approval to identify patients with locally advanced and metastatic non-small cell lung cancer eligible for Libtayo

Sanofi's Dupixent, amid €10B sales push, posts positive data in chronic spontaneous urticaria | Fierce Pharma
dvanced tment LIBTAYO: NOW APPROVED in locally advanced BCC and over 4 years of clinical treatment experience in advanced CSCC