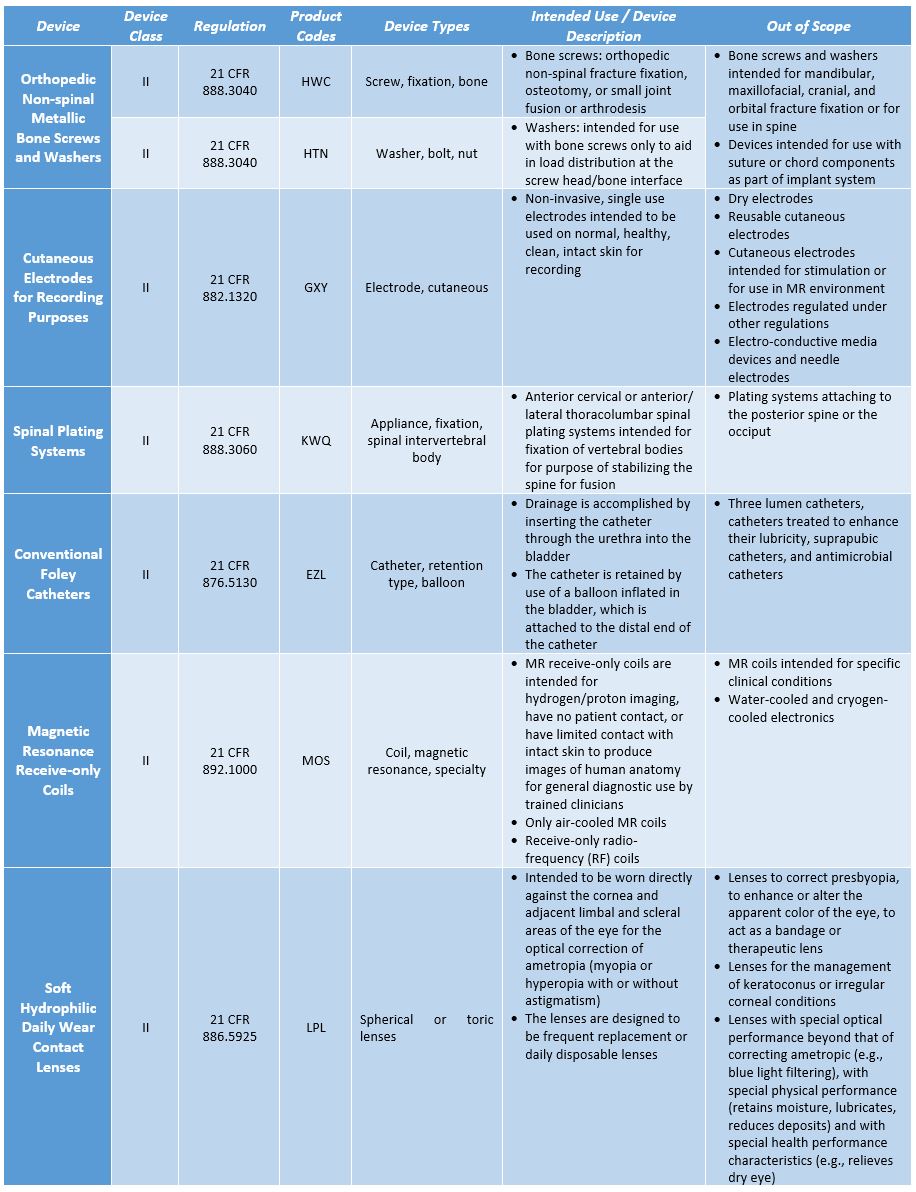
FDA's Safety And Performance-based Pathway An Alternative To Substantial Equivalence For 510(k) Submissions

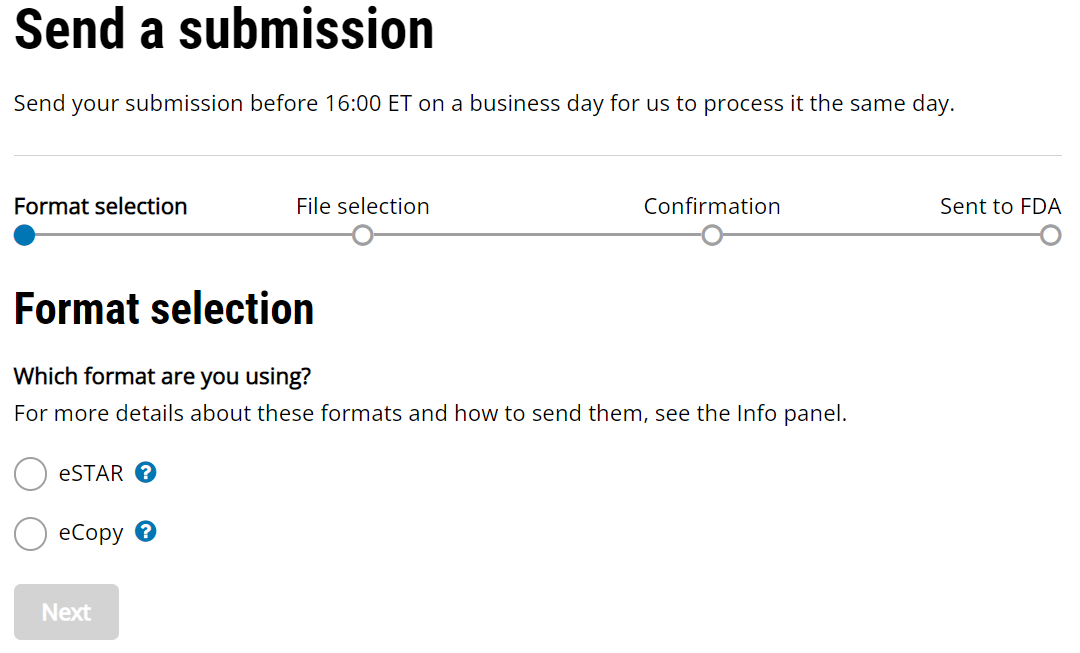
510k submission to the FDA (case study-part 1) Archives - Medical Device Academy Medical Device Academy

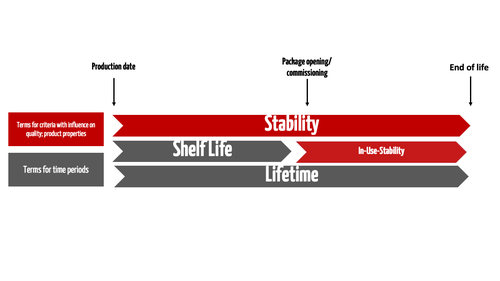
510k Submission, Section 14-Sterilization Validation and Shelf-life - Medical Device Academy Medical Device Academy

The 510(k) Program Roy Baby, Investigator US Food & Drug Administration 4040 N Central Expressway, Dallas, TX ppt video online download